Q1: Today’s question of the day is from the pre-algebra
If n = 1 – 2, what is 1/n + n3?
A. -2
Q2:
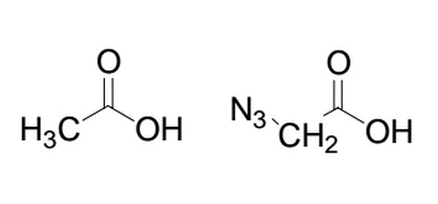
Given the two similar molecules below, which will have the lower pk a after deprotonation of the alcohol group and why?
 >
A
>
A
The left molecule because the carbon stabilizes the
conjugate base better than the nitrogen group
B
The left molecule because the nitrogen group is electron-
withdrawing, which stabilizes the negatively charged
conjugate base
C
The right molecule because the nitrogen group will
accept a proton, stabilizing the conjugate base
D
The right molecule because the nitrogen group is
electron-withdrawing, which stabilizes the negatively charged conjugate base
If n = 1 – 2, what is 1/n + n3?
A. -2
B. -1
C. 0
D. 1
E. 2
Q2:Given the two similar molecules below, which will have the lower pk a after deprotonation of the alcohol group and why?
 >
>The left molecule because the carbon stabilizes the
conjugate base better than the nitrogen group
B
The left molecule because the nitrogen group is electron-
withdrawing, which stabilizes the negatively charged
conjugate base
C
The right molecule because the nitrogen group will
accept a proton, stabilizing the conjugate base
D
The right molecule because the nitrogen group is
electron-withdrawing, which stabilizes the negatively charged conjugate base
No comments:
Post a Comment